RTI Surgical Implants
RTI Surgical Implants
Structural Allografts
Whole bones are used as a sterile source of cortical and cancellous bone. These implants may also be used in procedures that restore segmental and critical bone loss in the extremities due to trauma, bone disease, or from the removal of tumors and/or hardware. After being processed through RTI's BioCleanse® Tissue Sterilization Process, they are terminally sterilized in their final packaging to achieve a sterility assurance level (SAL) of 10-6.
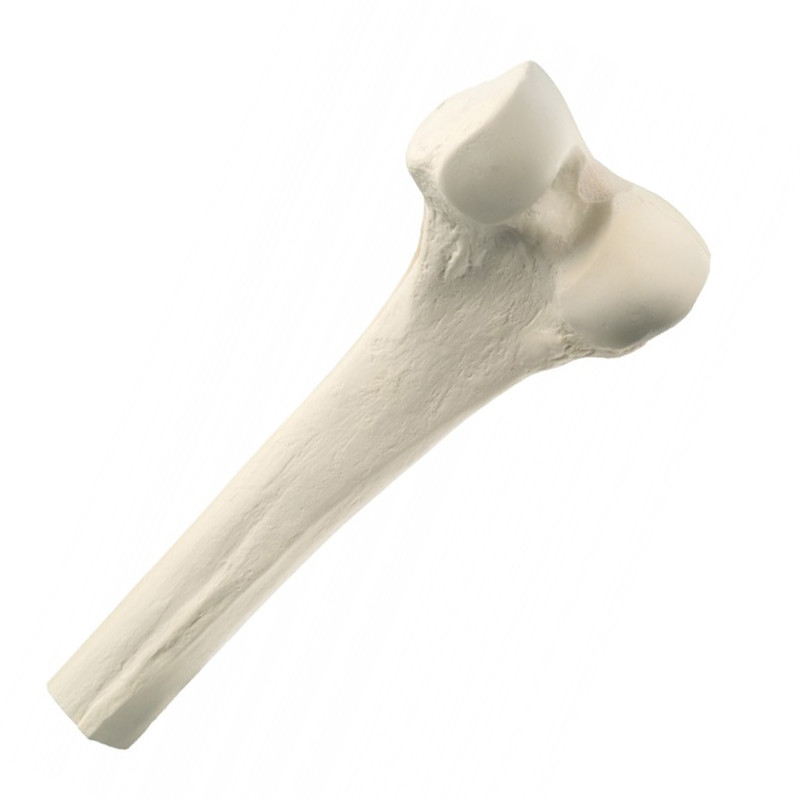
Overview & Features
PROXIMAL AND DISTAL GRAFTS
Sterilized through the BioCleanse® Tissue Sterilization Process
- Preservation: Frozen
- Terminal sterilization after the BioCleanse Process achieves SAL 10-6 and grafts are labeled STERILE
- Existing cartilage still attached
Implants
- Proximal Femur with Head
- Distal Femur
- Proximal Humerus
- Distal Humerus
- Proximal Tibia
- Distal Tibia
WHOLE BONES
Sterilized through the BioCleanse® Tissue Sterilization Process
- Preservation: Frozen
- Terminal sterilization after the BioCleanse Process achieves SAL 10-6 and grafts are labeled STERILE
- Existing cartilage still attached
- Variable lengths
Implants
- Whole Femur
- Whole Humerus
- Whole Tibia
- Whole Fibula
- Whole Radius
- Whole Ulna
- Distal Tibia

The BioCleanse Tissue Sterilization Process is an automated, pharmaceutical-grade process that sterilizes grafts that provide a natural biologic scaffold in orthopedic, spine and sports medicine procedures.
Regulatory approvals vary by country. Therefore, we kindly ask you to contact the distributor in your region regarding availability of specific products, implants and / or instrumentation in your region.
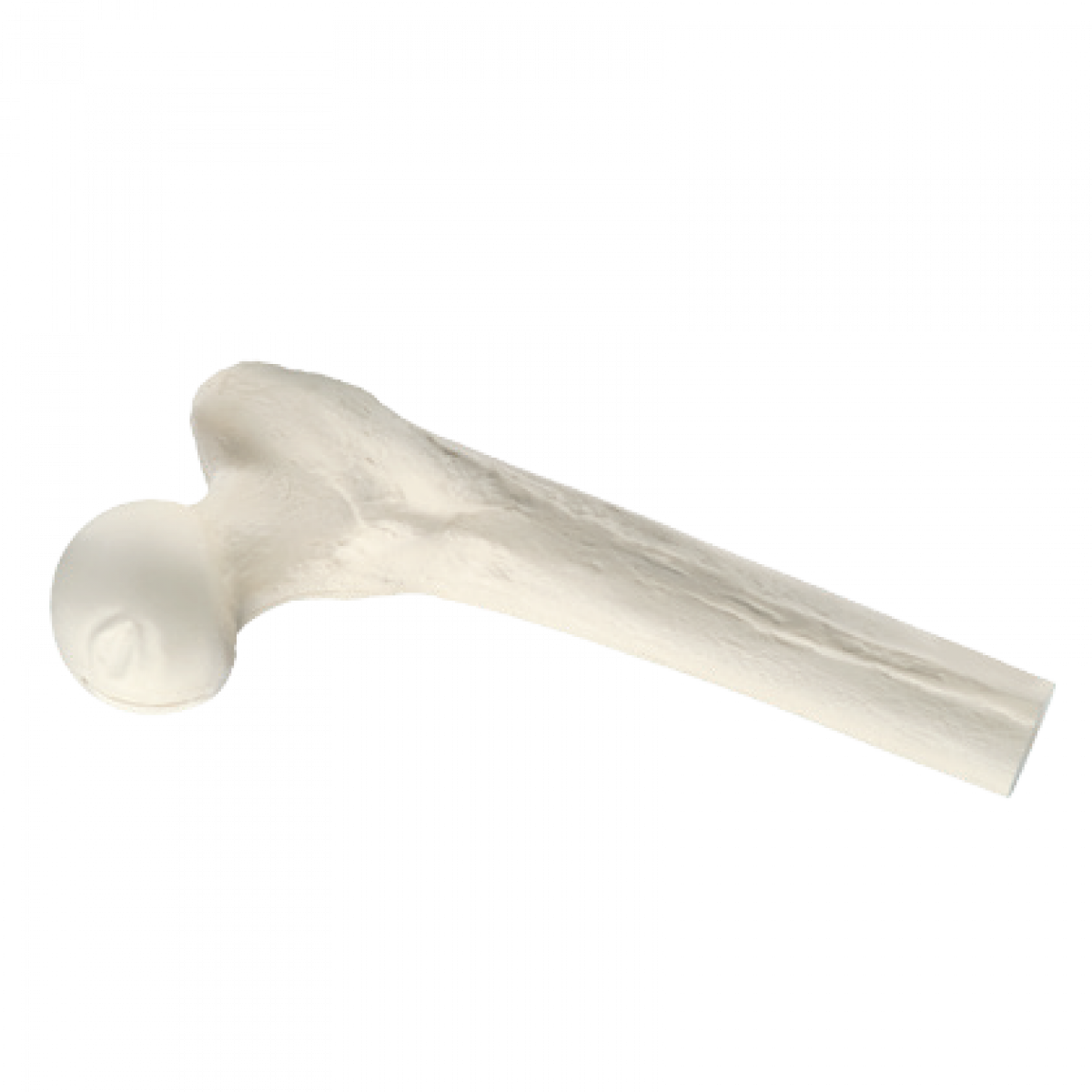
Proximal Femur with Head
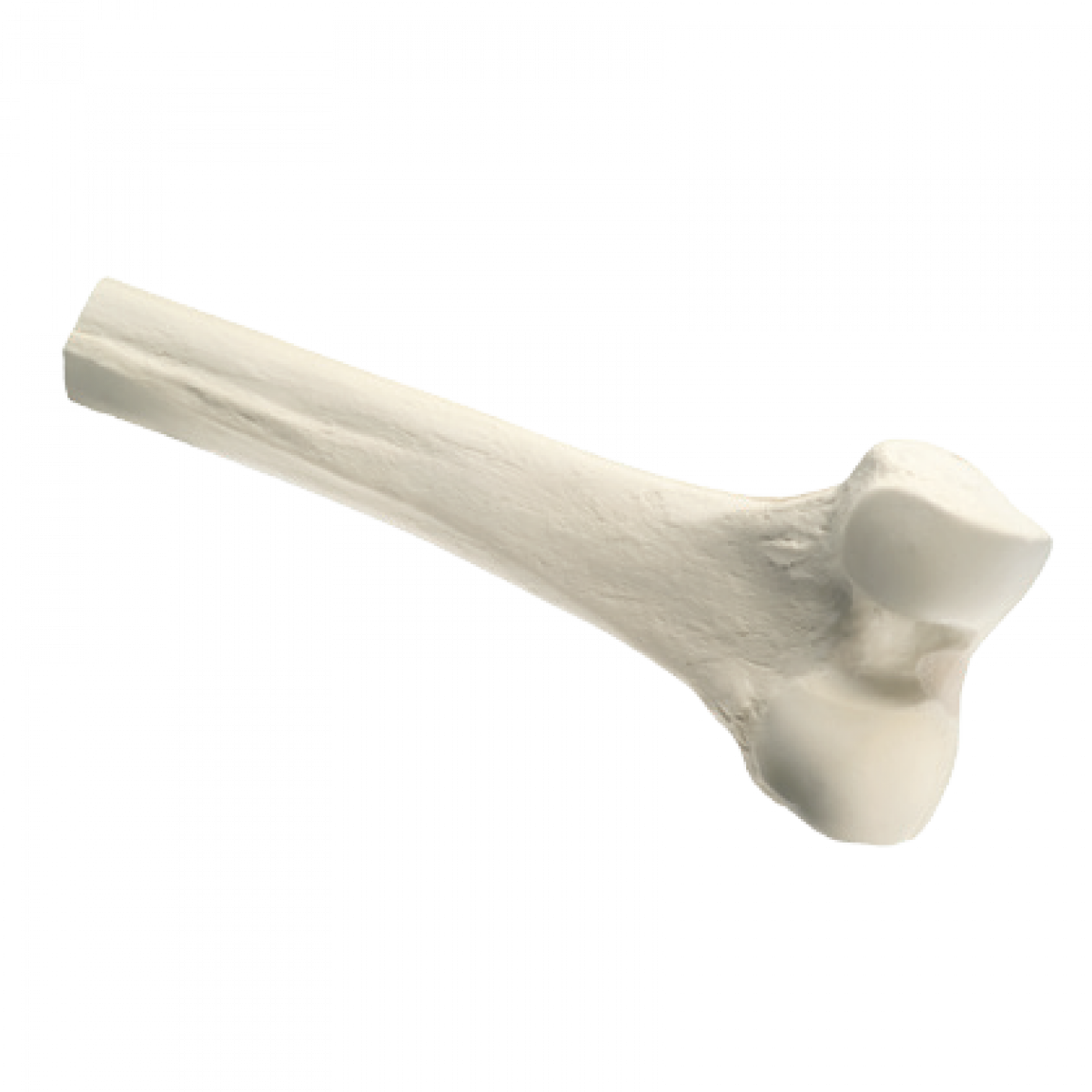
Distal Femur
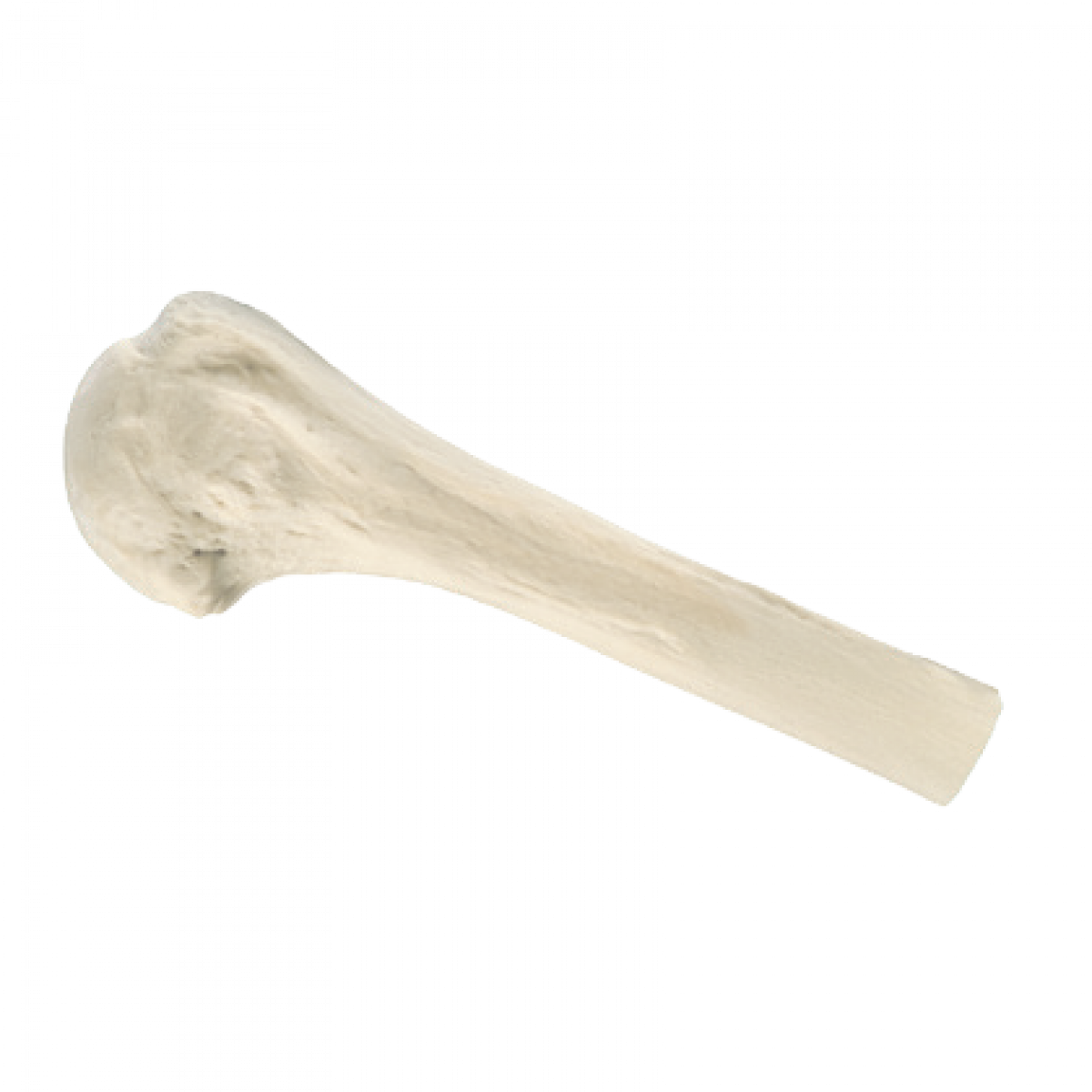
Proximal Humerus
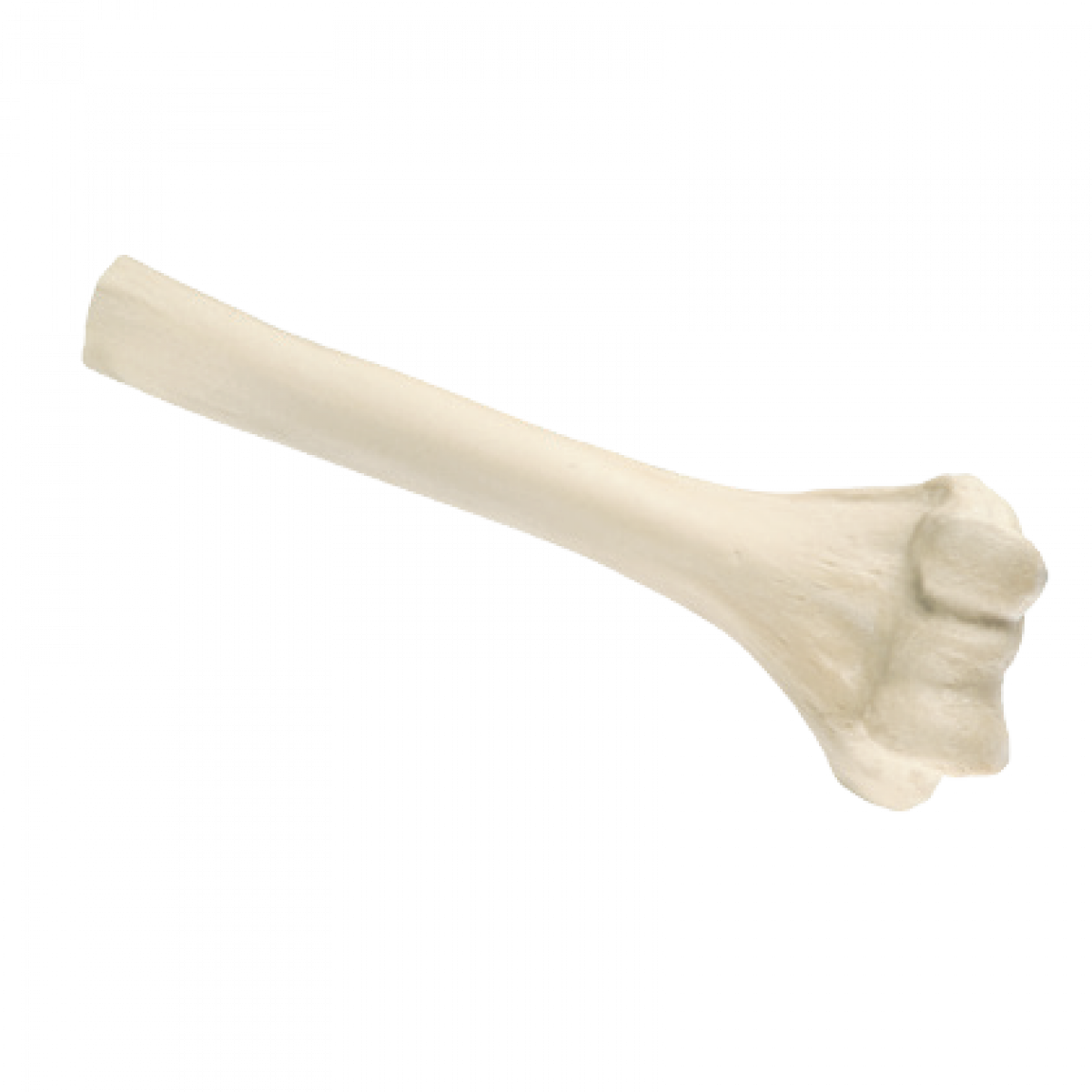
Distal Humerus
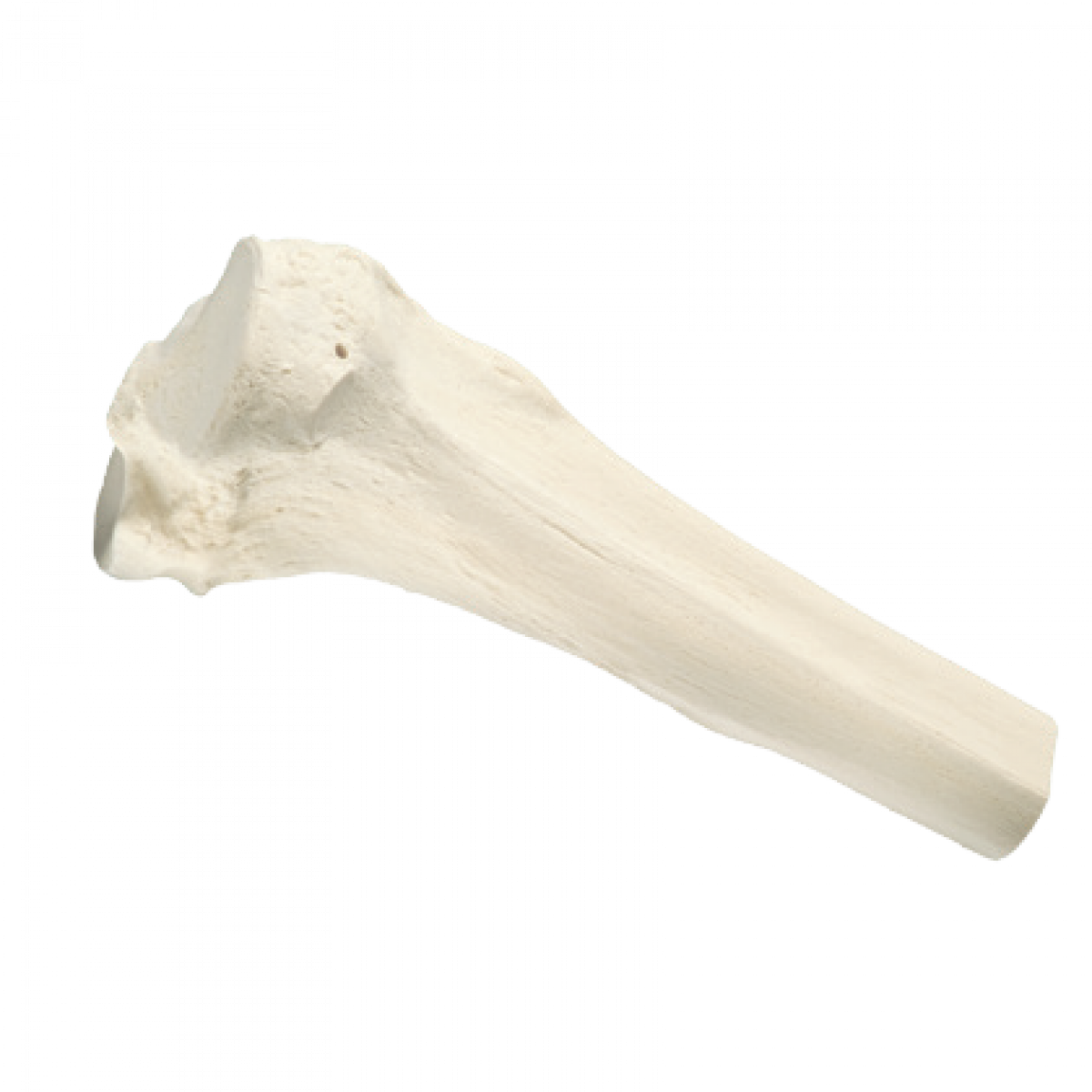
Proximal Tibia

Distal Tibia
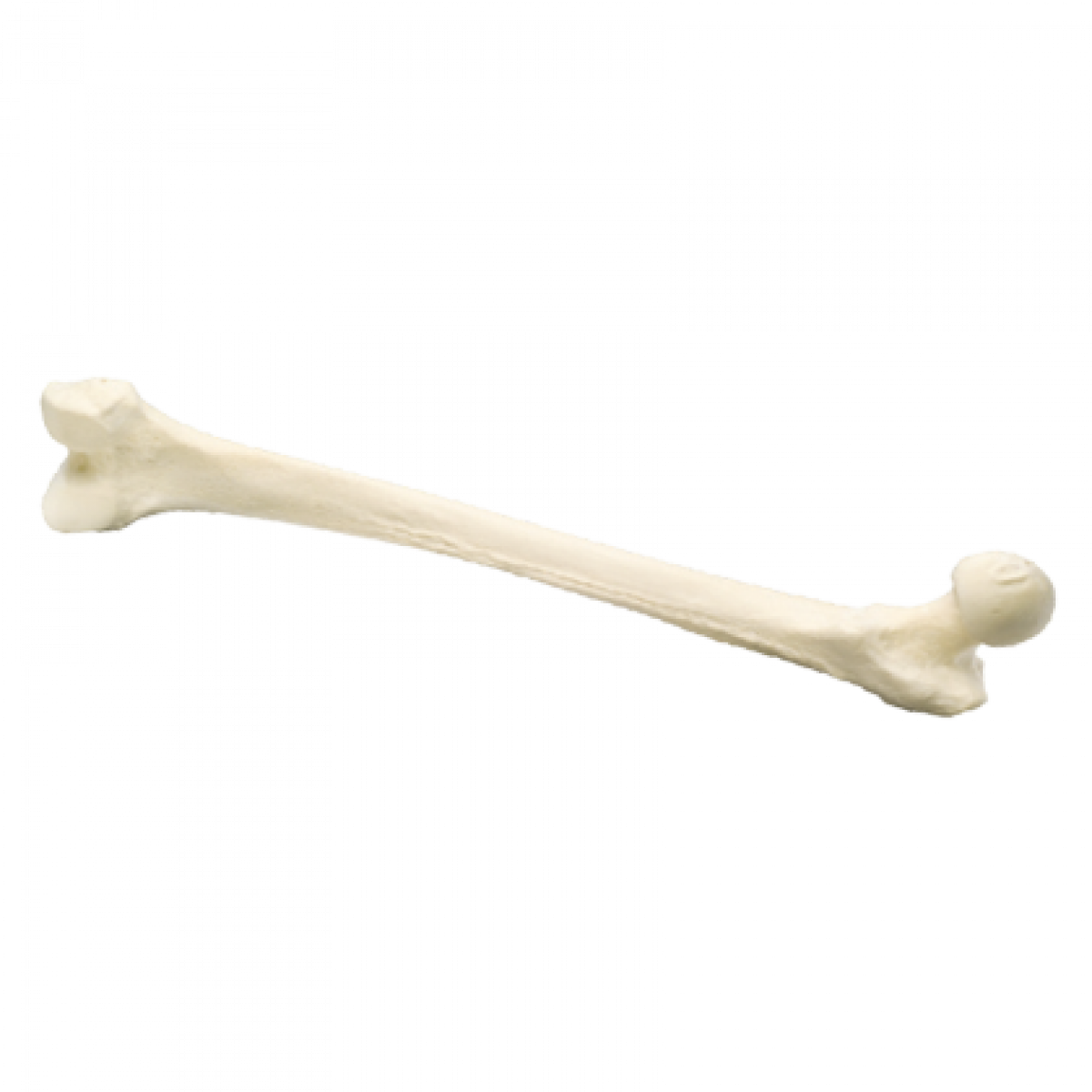
Whole Femur
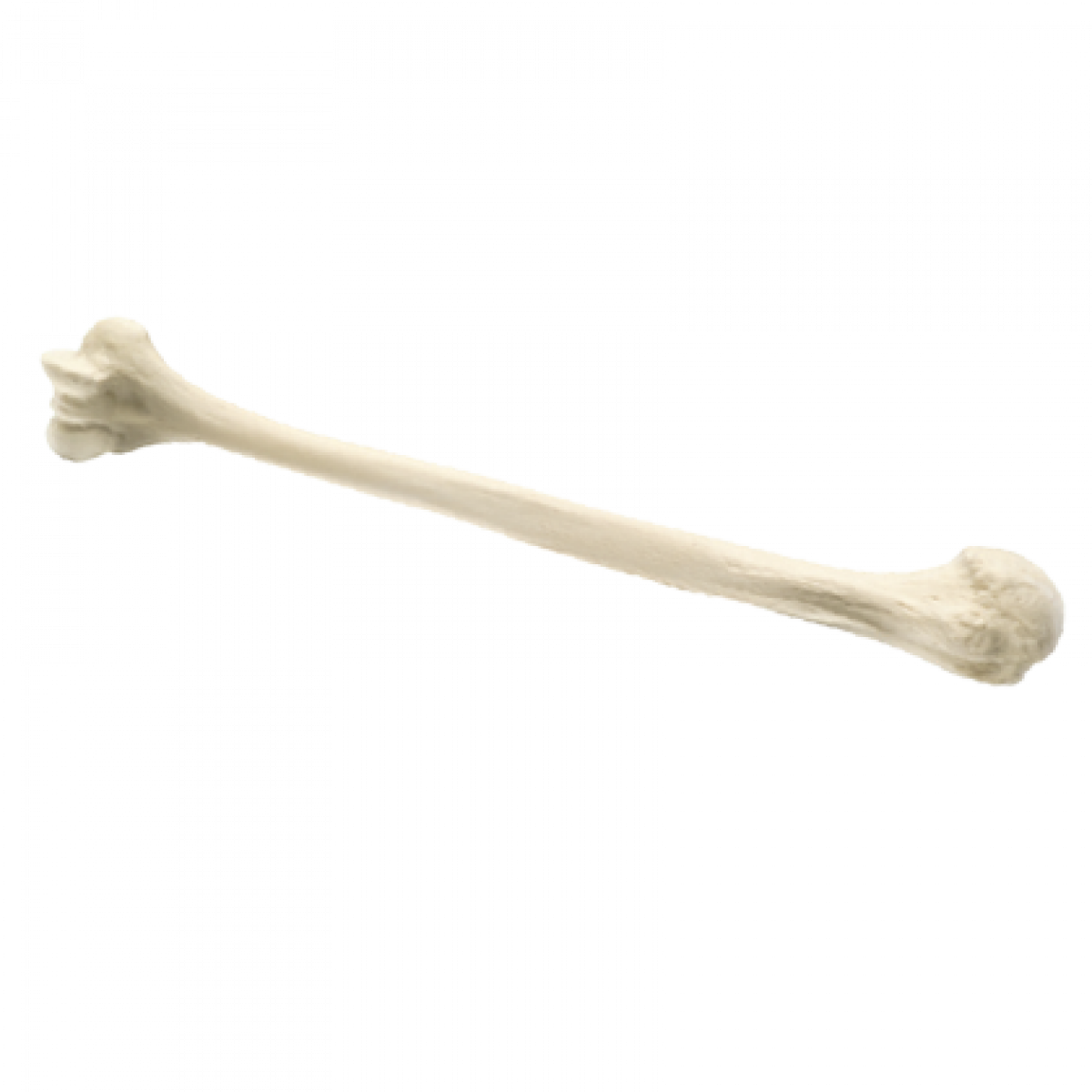
Whole Humerus
Whole Tibia

Whole Fibula

Whole Radius

Whole Ulna