Labeling
GS1 Global Trade Item Number (GTIN) Search
For your convenience, GS1 GTINs are available here for those RTI products that have been assigned GTINs. To accurately identify a product, refer to the product label for the product description or catalog number (REF). Email Labeling@rtix.com with any questions.
No search results for {{ query }}
| Product Name | Model/Version # | Description | GTIN |
|---|---|---|---|
Symbols in Labeling
| Symbol | Title | Definition | Standard | Reference Number |
|---|---|---|---|---|
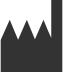 |
Manufacturer | Indicates the medical device manufacturer, as defined in EU Directives 90/385/EEC, 93/42/EEC and 98/79/EC. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied |
5.1.1 |
 |
Authorized Representative in the European Community | Indicates the Authorized Representative in the European Community. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.1.2 |
 | Authorized representative in Switzerland | Indicates the authorized representative in Switzerland | ISO 15223-1:2021, ISO 3166-1 | 5.1.2 |
 | Authorized representative in the United Kingdom | Indicates the authorized representative in the United Kingdom | ISO 15223-1:2021, ISO 3166-1 | 5.1.2 |
 |
Date of Manufacture | Indicates the date of manufacture. This symbol is accompanied by a date. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.1.3 |
 |
Use-by Date | Indicates the date after which the medical device is not to be used. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.1.4 |
 |
Batch Code | Indicates the manufacturer's batch code so that the batch or lot can be identified NOTE: Synonyms for "batch code" are "lot number" and "batch number" | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.1.5 |
 |
Catalog Number | Indicates the manufacturer's catalog number so that the medical device can be identified NOTE: Synonyms for "catalogue number" are "reference number" and "reorder number" | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.1.6 |
 |
Serial Number | Indicates the manufacturer's serial number so that a specific medical device can be identified | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.1.7 |
 |
Sterile | Indicates a medical device that has been subjected to a sterilization process |
ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.2.1 |
 |
Sterilized Using Aseptic Processing Techniques | Indicates a medical device that has been sterilized using aseptic processing techniques. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.2.2 |
 |
Sterilized Using Ethylene Oxide | Indicates a medical device that has been sterilized using ethylene oxide. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.2.3 |
 |
Sterilized Using Irradiation | Indicates a medical device that has been sterilized using irradiation. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.2.4 |
 |
Do Not Resterilize | Indicates a medical device that is not to be resterilized. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.2.6 |
 |
Non-Sterile | Indicates a medical device that has not been subjected to a sterilization process. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.2.7 |
 |
Do Not Use if Package is Damaged | Indicates a medical device that should not be used if the package has been damaged or opened. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.2.8 |
 |
Keep Away from Sunlight | Indicates a medical device that needs protection from light sources. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.3.2 |
 |
Keep Dry | Indicates a medical device that needs to be protected from moisture. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.3.4 |
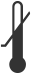 |
Lower Limit of Temperature | Indicates the lower limit of temperature to which the medical device can be safely exposed. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.3.5 |
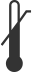 |
Upper Limit of Temperature | Indicates the upper limit of temperature to which the medical device can be safely exposed. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.3.6 |
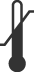 |
Temperature Limit | Indicates the temperature limits to which the medical device can be safely exposed. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.3.7 |
 |
Do Not Reuse | Indicates a medical device that is intended for one use, or for use on a single patient during a single procedure. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.4.2 |
 |
Consult Instructions For Use |
Indicates the need for the user to consult the instructions for use. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.4.3 |
 |
Electronic IFU indicator | Consult instructions for use for an electronic instruction for use | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | A.15 |
 |
Caution | Indicates the need for the user to consult the instructions for use for important cautionary information such as warnings and precautions that cannot, for a variety of reasons, be presented on the medical device itself. | ISO 15223-1 Medical Devices – Symbols To Be Used with Medical Device Labels, Labeling, and Information to be Supplied | 5.4.4 |
 |
Contains Or Presence Of | To indicate that the equipment contains the identified product or substance. | ISO 7000/IEC 60417 Graphical Symbols for Use on Equipment | 2725 |
 | UKCA marking | To demonstrate compliance with UK regulations of devices placed on the market in Great Britain | UK MDR SI 2002 No 618 | |
 | Self-Tapping Screw | To indicate self-tapping screw | N/A, Resolve-created symbol | |
 | Self-Drilling Screw | To indicate self-drilling screw | N/A, Resolve-created symbol |
U.S. Food and Drug Administration (FDA) Unique Device Identification Rule
The FDA issued a “final rule” (September 24, 2013) to establish a system to adequately identify medical devices through distribution and use. This rule requires the label of medical devices to include a unique device identifier (UDI), except where the rule provides for an exception or alternative placement. The UDI system will go into effect in stages, over a period of seven years. RTI has established a team to ensure compliance with the UDI rule within the specified timeframe. We have addressed some of the common questions below. However, if you have additional questions or need more information about the UDI transition, send your questions to Labeling@rtix.com.
What does the UDI rule include?
- The label of a medical device is required to bear a UDI.
- Any date on the label that should be brought to the attention of the user e.g., expiration date, date of manufacture, must be presented in the format four digit year – two digit month – two digit day (e.g., 2016-09-24).
- Labelers are required to submit certain data about devices to the Global Unique Device Identifier Database (GUDID). The GUDID is administered by the FDA.
- Devices intended to be used more than once and reprocessed before each use are required to bear a permanent UDI affixed to the device itself unless an exception applies.
What is a UDI?
- A UDI is a numeric or alphanumeric identification code assigned to medical devices by a labeler of the device. A unique device identifier is typically comprised of two segments; a device identifier (DI) and production identifier (PI).
- A DI is a mandatory, fixed portion of the UDI that identifies the labeler and the specific model or version of the device e.g., Global Trade Item Number (GTIN)
- A PI is a conditional, variable portion of the UDI e.g., lot number
Which RTI products are affected by the UDI requirements?
The UDI rule applies to all medical devices. Most of RTI’s products are medical devices and subject to the UDI rule but most of the human tissue RTI processes are not medical devices and the UDI rule does not apply.
Will the GTIN be used for traceability?
For those items labeled with a GTIN, the GTIN will accurately identify the model/ version number and the labeler of that particular item. For traceability, end users should capture the GTIN and the production data/ identifiers, applicable for the specific item in question e.g. serial number, expiration date, lot number.
When a customer places an order, will anything change? Can a customer still use catalog codes?
Customers will continue to use RTI catalog codes. There will be no immediate changes to packing slips and invoices.
What should customers do with existing RTI inventory? Do we need to send inventory back to RTI for relabeling?
Devices that are in commercial distribution prior to the applicable compliance date do not have to comply with the final rule.
When will UDI be implemented?
The UDI system will go into effect in stages. The table below lists the various compliance dates originally published by the FDA. The table below does not take into account the various extensions and exceptions provided for different device classes.
| Compliance Date | Requirement |
|---|---|
| 1 year after publication of the final rule (September 24, 2014) | The labels and packages of class III medical devices and devices licensed under the Public Health Service Act (PHS Act) must bear a UDI. § 801.20. Dates on the labels of these devices must be formatted as required by § 801.18. Data for these devices must be submitted to the GUDID database. § 830.300. A 1-year extension of this compliance date may be requested under § 801.55; such a request must be submitted no later than June 23, 2014. Class III stand-alone software must provide its UDI as required by § 801.50(b). |
| 2 years after publication of the final rule (September 24, 2015) | The labels and packages of implantable, life-supporting, and life-sustaining devices must bear a UDI. § 801.20. Dates on the labels of these devices must be formatted as required by § 801.18. |
| A device that is a life-supporting or life-sustaining device that is required to be labeled with a UDI must a bear UDI as a permanent marking on the device itself if the device is intended to be used more than once and intended to be reprocessed before each use. § 801.45. Stand-alone software that is a life-supporting or life-sustaining device must provide its UDI as required by § 801.50(b). | |
| Data for implantable, life-supporting, and life-sustaining devices that are required to be labeled with a UDI must be submitted to the GUDID database. § 830.300. | |
| 3 years after publication of the final rule (September 24, 2016) | Class III devices required to be labeled with a UDI must bear a UDI as a permanent marking on the device itself if the device is a device intended to be used more than once and intended to be reprocessed before each use. § 801.45. |
| The labels and packages of class II medical devices must bear a UDI. § 801.20. Dates on the labels of these devices must be formatted as required by § 801.18. Class II stand-alone software must provide its UDI as required by § 801.50(b). | |
| Data for class II devices that are required to be labeled with a UDI must be submitted to the GUDID database. § 830.300. | |
| 5 years after publication of the final rule (September 24, 2018) | A class II device that is required to be labeled with a UDI must bear a UDI as a permanent marking on the device itself if the device is a device intended to be used more than once and intended to be reprocessed before each use. § 801.45. |
| The labels and packages of class I medical devices and devices that have not been classified into class I, class II, or class III must bear a UDI. § 801.20. Dates on the labels of all devices, including devices that have been excepted from UDI labeling requirements, must be formatted as required by § 801.18. | |
| Data for class I devices and devices that have not been classified into class I, class II, or class III that are required to be labeled with a UDI must be submitted to the GUDID database. § 830.300. Class I stand-alone software must provide its UDI as required by § 801.50(b). | |
| 7 years after publication of the final rule (September 24, 2020) | Class I devices, and devices that have not been classified into class I, class II, or class III that are required to be labeled with a UDI, must a bear UDI as a permanent marking on the device itself if the device is a device intended to be used more than once and intended to be reprocessed before each use. § 801.45. |
Contact Us
Email Labeling@rtix.com with any questions.
About
Contact Us
Email Labeling@rtix.com with any questions.