RTI Surgical Implants
RTI Surgical Implants
RapidEase™ Pre-Sutured Tendon
Designed with the patient and surgeon in mind — minimizing graft preparation time and eliminating the potential complications associated with donor site autograft recovery.
The RapidEase™ Pre-Sutured Tendon (PST) is a tendon construct for use in Anterior Cruciate Ligament (ACL) and Posterior Cruciate Ligament (PCL) reconstruction procedures. RapidEase PST is available in single and quadruple strand configurations and sutured using sterile, ultra-high molecular weight polyethylene (UHMWPE) nonabsorbable sutures.
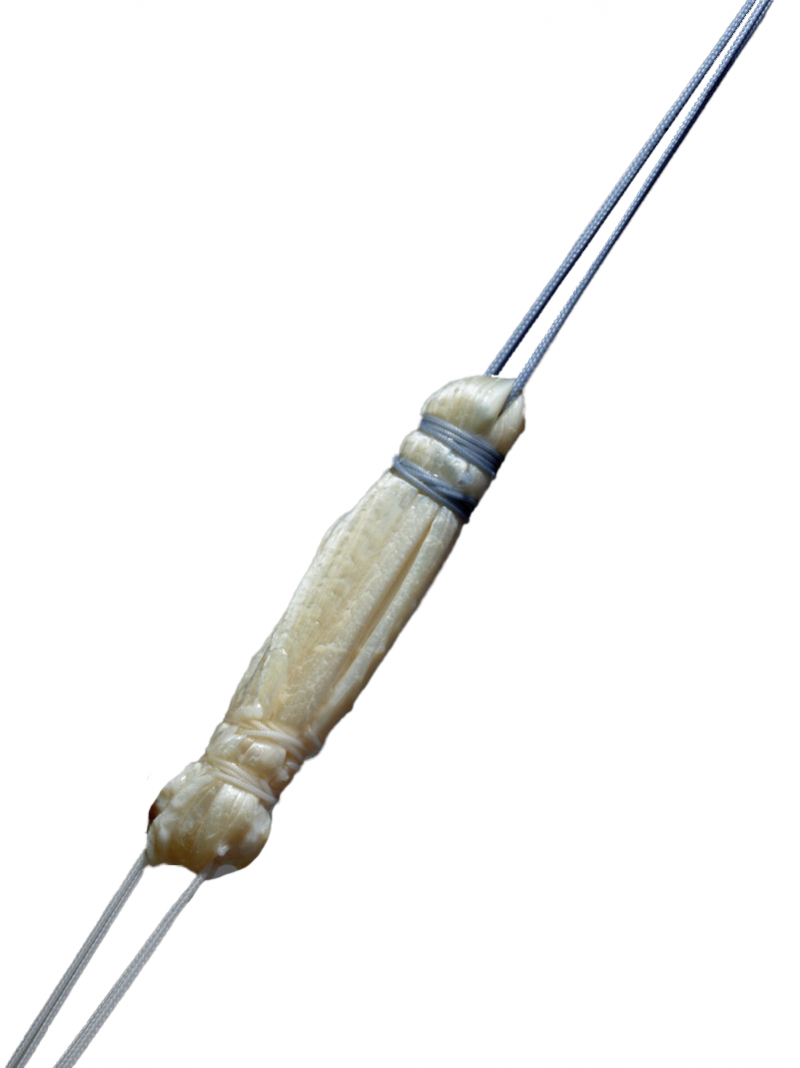
Overview & Features
Key Implant Features
• Safety: BioCleanse® processed to achieve a Sterility Assurance Level (SAL) of 10 -6 then aseptically sutured and packaged to assess graft quality.
» The BioCleanse process is validated to achieve microbial kill and viral inactivation using a worst-case biological indicator and virus panel.
• Quality: Every RapidEase PST is carefully measured, accurately sized, and stringently inspected for visual defects such as fiber separation and tendinopathy.
• Predictability: RTI's skilled processors undergo a robust qualification program to ensure consistent processing and suitable biomechanical strength across all [RapidEase PST] constructs.
Regulatory approvals vary by country. Therefore, we kindly ask you to contact the distributor in your region regarding availability of specific products, implants and / or instrumentation in your region.