RTI Surgical Implants
RTI Surgical Implants
Fresh-Stored Osteochondral Grafts
RTI Surgical’s fresh-stored osteochondral (OC) allografts enable surgeons to resurface osteochondral defects with mature hyaline cartilage and repair subchondral bone in a single procedure. Fresh grafts are cleansed, processed and preserved to maintain chondrocyte viability.

Overview & Features
FRESH GRAFTS
Aseptically Processed
- Preservation: Refrigerated
- Specifically sized to match patient radiographs or MRIs
- RTI’s process for fresh OC grafts addresses tissue safety while maintaining chondrocyte viability through all phases of pre-transplantation screening, testing and processing in accordance with applicable regulations and standards. RTI accomplishes this via:
- Rigorous pre-transplantation donor screening and testing
- Validated processing techniques
- Highly skilled and trained operators
- Qualified shipping measures
- Stringent visual inspection and microbiological culturing criteria
- Aseptic processing in a certified ISO Class 5 clean environment to ensure a high-quality processing setting
- Proprietary antibiotic soak developed by RTI to reduce potential contamination
- Eight cultures are taken during processing and final packaging episodes to detect microbial contamination
- Refrigeration in nutrient media to preserve chondrocyte viability
- Validated packaged shelf life of 45 days to preserve chondrocyte viability
- Fresh OC grafts are released for distribution after 14 days of culture monitoring, although final fungal cultures continue to be monitored for a total of 28 days
Implants
- Fresh-stored OC Femoral Condyle
- Fresh-stored OC Talus
- Fresh-stored OC Humeral Head
- Fresh-stored OC Femoral Distal Tibia
- Fresh-stored OC Femoral Trochlea
- Fresh-stored OC Femoral Patella
PRECISION ALLOGRAFT CARTILAGE KIT™ (PACK)
The Precision Allograft Cartilage Kit: An optimized fit for your articular cartilage resurfacing cases.
PACK is designed to minimize trauma to the allograft caused by the technique.
- Cutters provide a clean recipient socket and the cutting tools leave a pristine plug surface.
- Comprehensive range of sizes helps to achieve an optimized fit. Coring reamers and tamps are available from 12 - 35mm in 2mm increments.
- Clear ring guides provide visibility
- The design minimizes the force needed to remove the allograft plug from the reamer.
- With the cylindrical ruler design, the surgeon can see the measurements from any angle when measuring the patient’s socket and the allograft plug.
*Fresh-stored osteochondral allografts are cleansed, processed and preserved to maintain chondrocyte viability, and therefore are not sterilized through the BioCleanse® or Tutoplast® Tissue Sterilization Processes. Please refer to the labeling for clinical applications, warnings, precautions and other instructions for use.
Regulatory approvals vary by country. Therefore, we kindly ask you to contact the distributor in your region regarding availability of specific products, implants and / or instrumentation in your region.
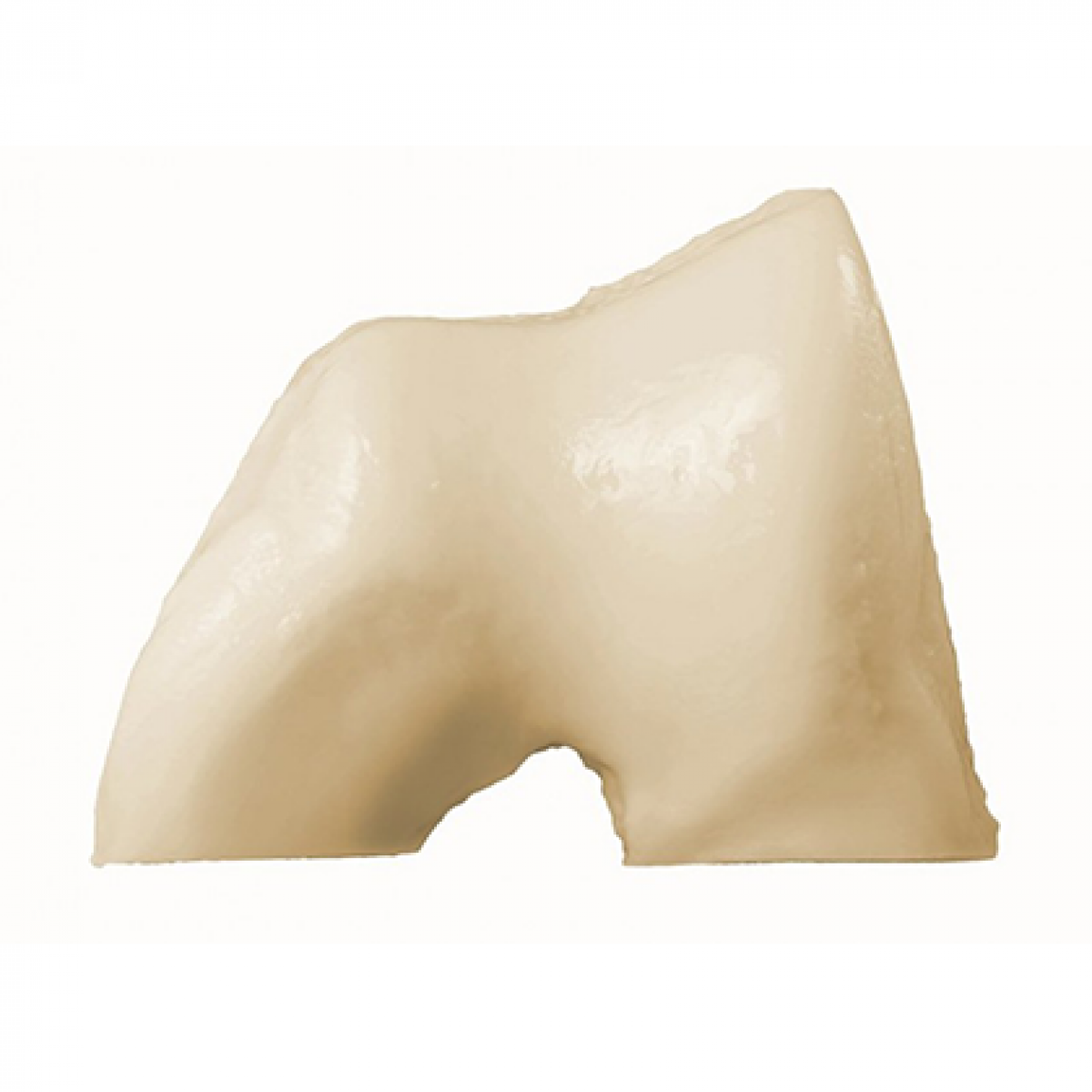
Fresh-Stored OC Trochlea

Fresh-Stored OC Patella
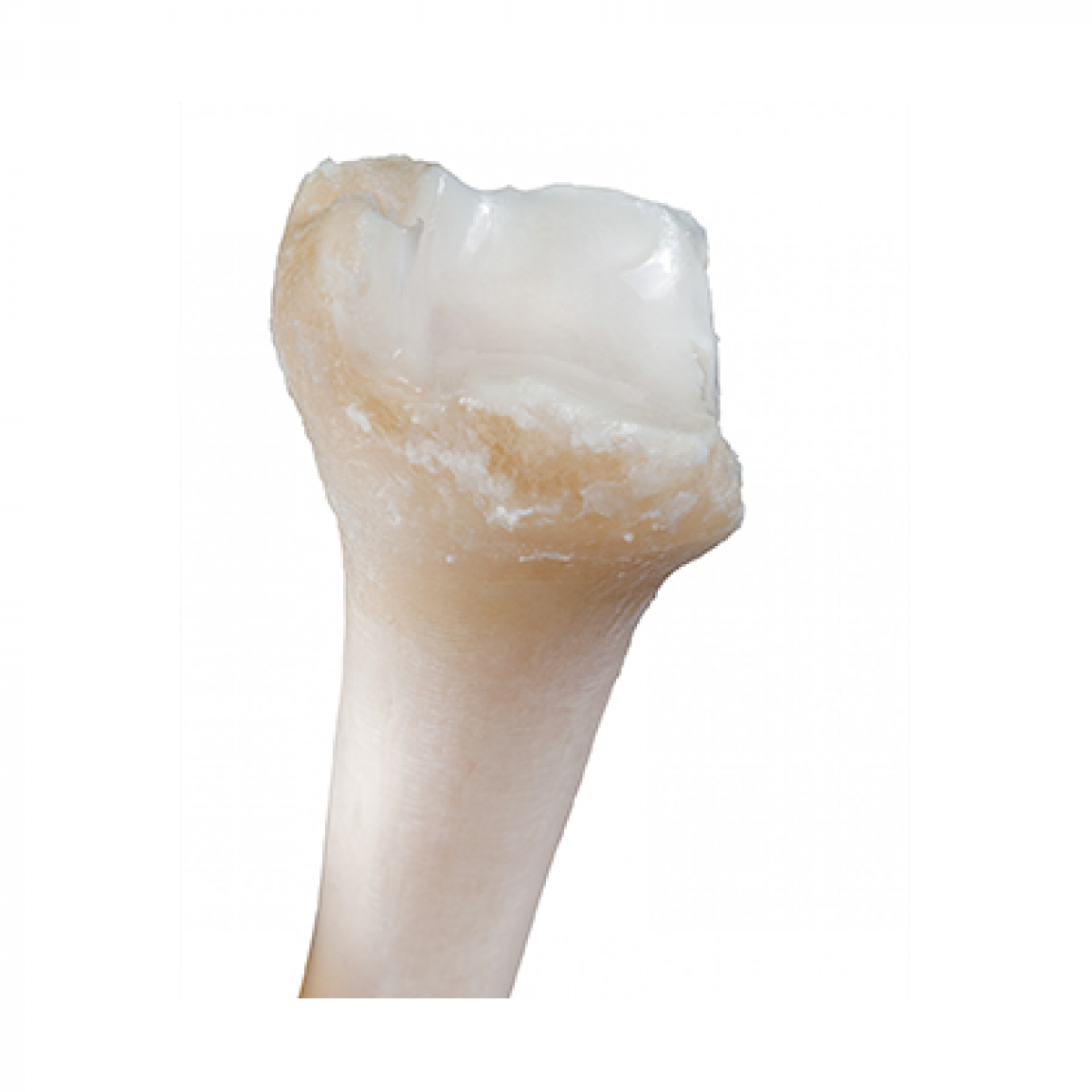
Fresh-Stored OC Distal Tibia
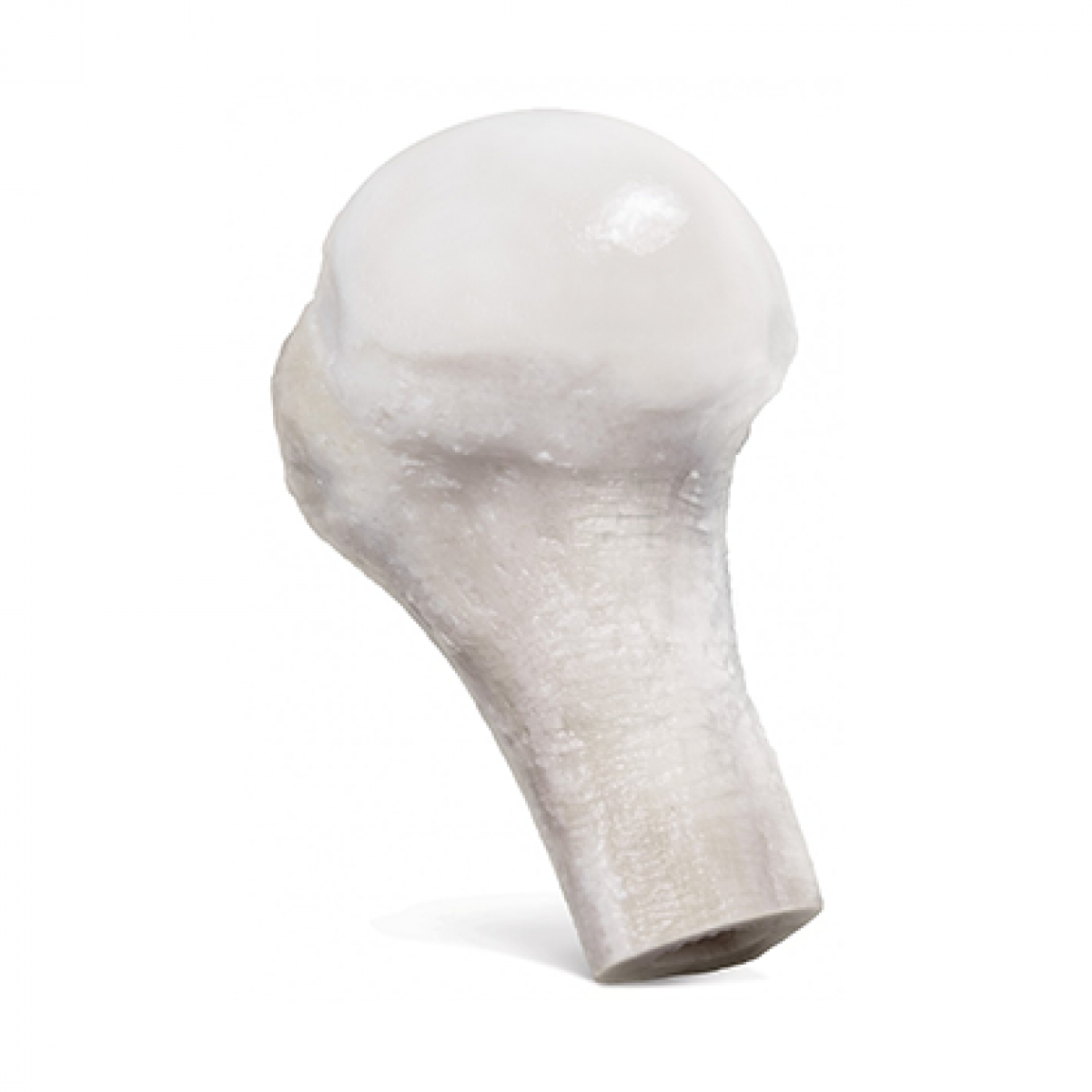
Fresh-Stored OC Humeral Head

Fresh-Stored OC Femoral Condyle
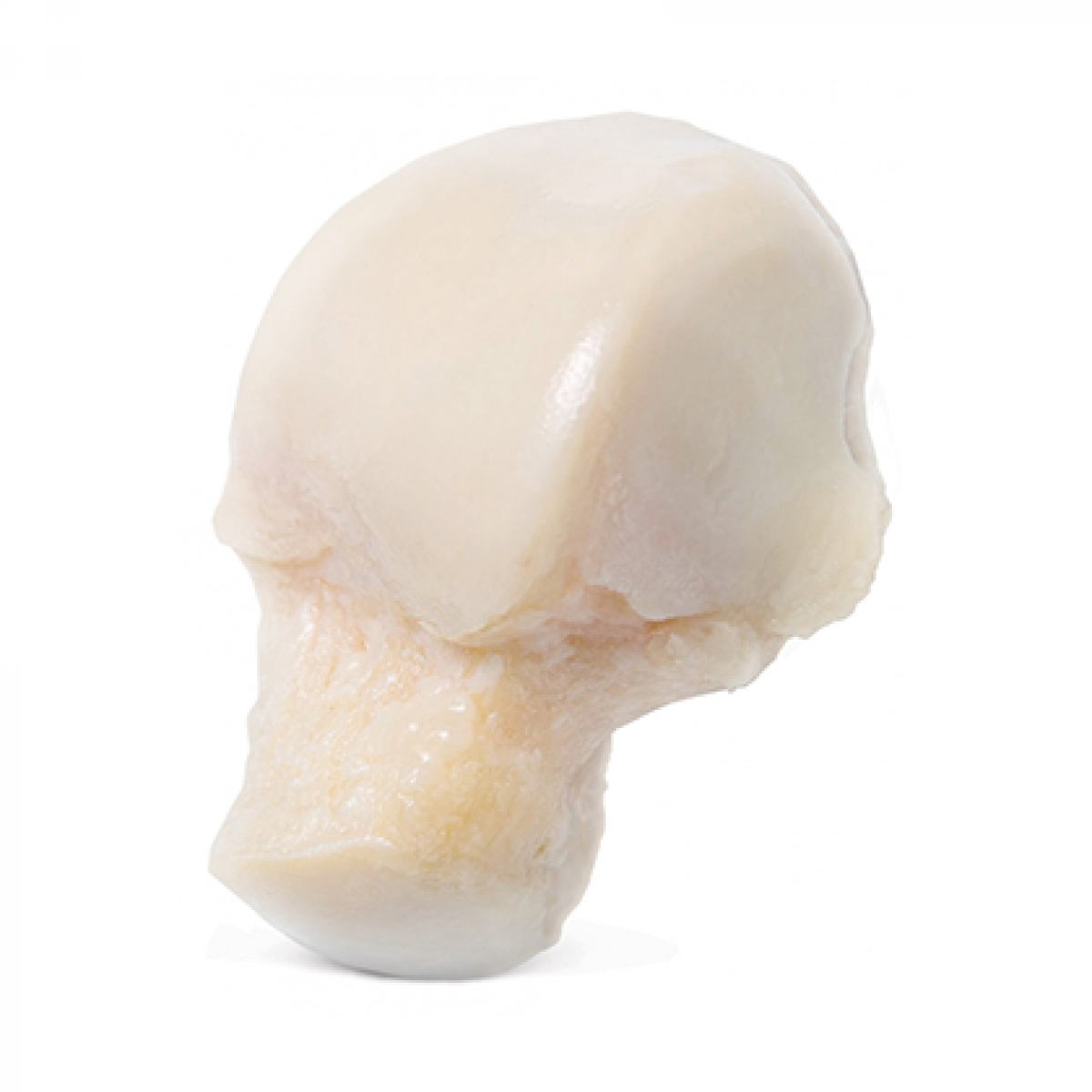
Fresh-Stored OC Talus

Precision Allograft Cartilage Kit (PACK)

Tamps

Vise

Sizing Block

Coupler

Coring Reamer and Caps